Organized by IRB Barcelona in collaboration with the Fundació Catalunya La Pedrera.
This year's tutors are Daniela Romão, Elena Gapar, Anna Pijuan, Maria Caballero, Mateusz Biesaga, Ilaria Dutto, Nevenka Radic, Diego Gallego, Miguel Martin, Marta Kovatcheva, Marina Bellido, Amir Ali, Blazej Baginski and Clara Borràs.
Presentation

Objectives
Crazy About Biomedicine is a course directed at students in their first year of baccalaureate who wish to explore some of the exciting discoveries being made in the life sciences. Through this course, students will have a chance to deepen their knowledge of scientific theory and techniques in the field of biomedicine. They will work alongside our young researchers to get a taste for what doing science in a top international research institute is like, gain some hands-on experience in the latest cutting-edge methodologies, and position themselves for a potential career in the life sciences.
Course Description
A year-long workshop in the life sciences for high-school students organized by IRB Barcelona within the series "Crazy About Science" of the Fundació Catalunya La Pedrera.
This course includes a combination of theoretical lectures and practical hands-on experimental activities, which take place on 18 Saturdays throughout the year. Presented by IRB Barcelona PhD students and postdocs, the course will cover 12 hot scientific topics, ranging from cell and molecular biology to structural and computational biology, and chemistry. In the first ‘semester’ (January-April), the first three Saturdays will be devoted to these general lectures for all participants. During the following six sessions, small groups will enter the labs for hands-on practical experience. This schedule will then be repeated with six new research topics in the second semester (May-November). Participants must commit themselves to attend the whole course.

Course language
All lectures and practical sessions will be conducted in English.
Course dates and times
The course will run from January to November 2020, 10.00-14.00h.
SEMESTER I
- Fri. 10 January 2020: Inauguration at Mon Sant Benet- Sant Fruitós del Bages
- Sat. 11 January: Talk 1-2
- Sat. 18 January: Talk 3-4
- Sat. 1 February: Talk 5-6
- Sat. 15 February: Practical session 1
- Sat. 29 February: Practical session 2
- Sat. 14 March: Practical session 3
- Sat. 28 March: Practical session 4
- Sat. 18 April: Practical session 5
- Sat. 25 April: Practical session 6
SEMESTER II
- Sat. 16 May: Talk 1-2
- Sat. 30 May: Talk 3-4
- Sat. 6 June: Talk 5-6
- Sat. 19 September: Practical session 1
- Sat. 3 October: Practical session 2
- Sat. 17 October: Practical session 3
- Sat. 24 October: Practical session 4
- Sat. 7 November: Practical session 5
- Sat. 21 November: Practical session 6
Course fees
There is a fee of 150 euros to contribute to administrative and insurance costs for this programme, payable directly to the Fundació Catalunya La Pedrera.
Course location
Institute for Research in Biomedicine (IRB Barcelona)
C/ Baldiri Reixac, 10
08028 Barcelona
Who can apply
This course is directed toward students in the first year of their baccalaureate (only), who have a special interest and talent in the fields related to the life sciences (primarily biology and chemistry).
Students may apply to a maximum of 2 programmes within the "Crazy About Science" series, and can participate in only one.
How to apply
 Applications have to be submitted from here, starting on 16 September 2019.
Applications have to be submitted from here, starting on 16 September 2019.
Interested students must fill in the online application form and include a letter of motivation. A letter of recommendation will be requested directly from two of their teachers, who should know the applicant well. If the applicant has recently changed school then the letters of recommendation should be requested from his/her former teachers.
The deadline for registration is 24 October 2019.
The course is open to a total of 24 students. Candidates will be selected on the basis of their academic record, teacher recommendations and motivation to participate. A shortlist of candidates will be invited for interviews with the scientific organizers in November after which the final selection will be made. Students will be informed of the outcome by the first week in December. The students selected to participate and their parents/guardians will be asked to sign a letter of commitment to attend all sessions.
Collaborators
Facebook: @LaPedrera.Fundacio
Twitter: @PedreraFundacio
Instagram: #bojosperlaciencia
Facebook: @LaPedrera.Ciencia
Twitter: @PedreraCiencia
Instagram: @lapedrera_ciencia

![]()
If you have any question, please contact us at irb_outreach@irbbarcelona.org
Important dates
 24 October 2019: Application deadline
24 October 2019: Application deadline- 7 November 2019: Short-listed candidates contacted
- 8-21 November 2019: Interviews
- 22 November 2019: Selected students contacted
- 10 January 2020: Inauguration at Món Sant Benet –Sant Fruitós del Bages
- 11 January 2020: Course begins
- Closing Ceremony - To be determined
Programme
SEMESTER 1
1. Interacting Is Living, Living Is Interacting
Diego Gallego (Molecular Modelling and Bioinformatics)

Have you ever wondered about the fundamental principles that explain life? Our body, a fruit fly, or a flower consists of cells. These (including bacteria and even archaebacteria), in turn, comprise four big groups of molecules: lipids, carbohydrates, proteins and nucleic acids. But if you mix these building blocks in vitro, you will not see a cell. And why not? Because a cell is much more than the sum of its parts. To understand the complete picture, you have to know how the parts (molecules) interact with each other. When you analyse networks of interactions, you can understand very complex processes such as transcription, translation, mitosis, metabolism, and signalling pathways, etc.
The correct interaction between any two molecules (e.g. a protein and DNA) depends heavily on their 3D structure. In turn, this structure is acquired through a process of folding, guided (again) by intra-molecular interactions. One of our main interests is understanding, modelling, and predicting the 3D structure, dynamics and interactions of nucleic acids.
In this course, we will see biomedicine from a computational perspective. We will work with a real modelling problem that will allow us to cross the boundaries between structural bioinformatics, chemistry and physics. After this experience, the take-home message will be clear: interactions are the fundamental principle that allows us to understand each and every process of life.
2. To Mutate or Not to Mutate?
Clara Borràs and Nevenka Radic (Signalling and Cell Cycle Laboratory)


Our cells can endure tens of thousands of DNA-damaging stimuli per day. However, not all DNA damage events will lead to a mutation because cells have evolved a number of mechanisms to recognise and repair various types of damage. When cells accumulate too much damage, they will either stop the cell cycle or die. However, certain cells “escape” this fate and continue dividing, a process that may lead to tumour formation.
In this course, we will talk about the importance of DNA repair mechanisms and the effect DNA damage can have on both healthy and tumour cells. We will also use a variety of techniques to determine the level of DNA damage in cancer cells under different conditions.
3. Identifying New Genes Involved in Brain Size Regulation
Ilaria Dutto (Genomic Instability and Cancer Laboratory)
![]()
To identify regulators of human brain size, we screened candidate genes that carried fixed sequence changes in H. sapiens compared to H. neanderthalensis. The screening identified a protein that plays a role in de novo synthesis of purine, one of the building blocks of nucleic acids (DNA and RNA). This protein is crucial for correct DNA replication and ATP/ADP ratio in the cell, and mutations in its sequence cause a disease characterised by a spectrum of central nervous system defects, including mental retardation, epilepsy, autism and microcephaly, as well as growth delay.
We use a human cell line to delineate the mechanisms at a cellular level, and zebrafish and chicken embryos to understand what defects protein depletion causes during development.
In this course, we will learn how identify an adequate model system for our research project, and how to culture human cell lines and prepare experiments to test cell proliferation.
4. Mirror, Mirror on the Wall… Who Is the Most Helpful Insect of Them All? – Getting to Know Tumorigenesis Through the Fly
Elena Gaspar and Daniela Ferreira (Development and Growth Control Laboratory)


The fruit fly Drosophila melanogaster is an impressive model organism that has a long history of helping researchers understand the basic processes behind several diseases and systemic behaviours. This tiny organism has emerged as a potent tool for genetic manipulation, offering innumerable possibilities to analyse the detailed interaction between cells and tissues.
On the molecular level, the fruit fly shares many similarities and conserved pathways with humans, and 60% of genes identified to be mutated, amplified or deleted in diverse human diseases have a counterpart in Drosophila.
During this semester, we will learn how to study the complex process of tumorigenesis using this model organism, both with a local and systemic approach. We will focus mainly on carcinomas, the most common type of tumour diagnosed in humans.
Carcinomas are derived from epithelial tissue, such as the skin, and they can become invasive or metastatic by spreading beyond the primary tissue layer and surrounding tissues or organs. In aggressive cancer cells, this transition is mediated by the activation of the EMT (Epithelial to Mesenchymal Transition) programme, which causes the cells to undergo morphogenetic alterations that increase invasive capacity.
In this course, we will introduce you to the first steps in fly genetics. We will cross flies, identify genetic markers and balancer chromosomes, and apply other genetic tools that we use in the lab every day. We will also learn about the anatomy of the fly in adult and larvae stages and use advanced microscopy to see several genetic markers. We will also perform in vivo dissections and their respective immunohistochemistry.
5. Proteins: Powerful Tools in Biomedicine
Mateusz Biesaga (Laboratory of Molecular Biophysics)

Proteins comprise a major part of living matter. They are key players in all cellular processes…they regulate, organise, transport, store, provide structure, and support other functions. Thanks to advances in biotechnology, scientists are now able to precisely design, produce and purify many proteins, thus allowing them to study the features of these molecules. Indeed, proteins are not only the focus of research activity but are also pivotal in many biomedical applications. One example is synthetic human insulin, which is produced in bacteria or yeast, used to treat millions of diabetes patients worldwide. A second example is the protein complex biotin and streptavidin, which is widely employed in molecular diagnostic tests.
In this course, we will learn how to produce proteins in vitro and understand their main structural and functional features. We will employ laboratory techniques that are used to obtain pure protein solutions. Furthermore, we will discuss how to engineer a protein, from the design of DNA that encodes it to the purification process, and also the use of recombinant proteins in biomedicine.
6. Dealing with Stress
Anna Pijuan and Maria Caballero (Cell Signaling)


A fundamental property of living cells is the ability to sense and respond to fluctuations in their environment. Cells have developed a number of signal transduction pathways that serve to adapt to and survive these changes.
The budding yeast Saccharomyces cerevisiae, a well-established eukaryotic model organism, has several advantages: ease of manipulation; short life span; ability to produce a large number of offspring; and a sequenced genome. We use S. cerevisiae to study the adaptive eukaryotic responses to a variety of environmental stresses. Remarkably, many processes initially discovered and studied in yeast, such as transcription regulation, stress-signalling transduction, and cell cycle regulation, are conserved in higher eukaryotes and therefore allow for translational studies.
Yeast and mammals have a conserved family of mitogen-activated protein kinase (MAPKs)—known as stress-activated signalling pathways (SAPKs) of the Hog1/p38 family—that sense and respond to changes in the extracellular environment. The activation of SAPKs leads to the generation of a set of adaptive responses that involves modulation of several physiological processes, such as changes in gene transcription, cell metabolism, protein translation and cell cycle progression.
In this course, we will learn how to manipulate and work with S. cerevisiae. To understand how cells detect an external signal and transduce it in order to elicit a proper response to ensure survival, we will study the activation of Hog1 MAPK at the protein level upon different stress conditions and also examine mutant strains. We will also learn how mutations in distinct genes can produce different phenotypes in response to stress.
SEMESTER 2
1. How Do Pro-Metastatic Fats Affect the Proteome?
Miguel Martin (Stem Cells and Cancer)
Cancer is a disease involving abnormal cell growth (tumour) with the potential to invade or spread to other parts of the body (metastasis). It is the fifth leading cause of death worldwide, and most cancer-related deaths are due to metastasis. Our group recently identified a group of cells responsible for initiating and promoting metastasis in several types of human tumour. These cells are characterised by the overexpression of the protein CD36, which absorbs fatty acids from the cell membrane. The metastatic process is enhanced by fat intake, and tumour cells produce more aggressive metastases in the presence of specific fatty acids, such as palmitic acid. In the absence of CD36, the tumours do not develop metastasis, or existing metastases shrink. Thus, blocking fat metabolism may provide an effective therapy to treat cancer patients.
Our research group is interested in why some specific fatty acids are pro-metastatic. One hypothesis is that these fatty acids (e.g. palmitic acid) can covalently attach to proteins and modify their function.
In this course, we will learn how to detect ‘palmitoylated’ proteins by metabolic labelling of proteins in cell culture with a palmitic acid-mimic compound.
2. Strategies to Understand Aging and Senescence
Marta Kovatcheva (Cellular Plasticity and Disease)

Scientific research and modern medicine have dramatically extended life expectancy, with the average person in the developed world expected to reach 80 years of age or more. However, this extension in lifespan has had no effect on health span; that is, the number of healthy years a human lives. Aging is still characterised by multiple pathologies including frailty, heart disease, cancer, and neurodegenerative diseases, among many others.
One of the main hallmarks of ageing is cellular senescence, the phenomenon by which normal cells stop dividing. Senescent cells accumulate in an organism over time, secreting pro-inflammatory molecules and contributing to age-related diseases. There is an increasing interest in clinical medicine to better identify and target senescent cells, as their elimination may delay and ameliorate some age-associated diseases.
This course provides hands-on experience using different techniques to induce cellular senescence in normal cells. We will learn state-of-the-art techniques to study the molecular biology of senescent cells, analysing in vitro and in vivo samples. Finally, we will perform classical protocols, such as SAβgal staining, to detect senescent cells. These techniques will help us to understand, identify and target senescent cells for clearance, which is a promising therapeutic approach to extend health span.
3. Microtubules: Essential Biopolymers for Cell Division
Aamir Ali (Microtubule Organization)

Analogous to the skeletal system that provides morphological structure to the human body, the cytoskeleton determines the shape of a cell. The cytoskeleton consists of the biopolymeric assemblies of microtubules, actin filaments, and intermediate filaments, which perform a variety of vital subcellular functions, such as the transport of biomolecules, cell division, organelle positioning, and cell polarization, etc.
Among the cytoskeleton components, microtubules are particularly interesting because of their essential role in cell division. Microtubules are made of alpha–beta–tubulin heterodimers and their assembly is precisely regulated in time and space during the cell cycle. During mitosis, microtubules assemble into a bipolar structure called mitotic spindle that mechanically segregates chromosomes into the daughter cells. The faithful segregation of the chromosomes is attributed to the physical interaction between the microtubule plus ends and a protein structure called kinetochores on the chromosomes. Subcellular depletion of a microtubule-associated CHTOG protein disrupts the microtubule–kinetochore interaction and leads to the unequal division of chromosomes, which is a hallmark of cancer progression.
In this course, we will study how perturbation of microtubules by depletion of CHTOG leads to unfaithful chromosome segregation. We will perform immunofluorescence-staining experiments in cultured osteosarcoma cells to visualise normal and abnormal chromosome segregation and gain insight into the cell division process.
4. Unravelling the Molecular Structure of Life
Blazej Baginski (Structural Characterization of Macromolecular Assemblies)

Proteins and nucleic acids (DNA and RNA) are the basic building blocks of life. They perform a multitude of functions–from sensing, transporting, and enzymatic regulation, to building the cell’s internal skeleton. Therefore, the fold and 3D-structure of these biomolecules is carefully controlled. A protein’s 3D structure determines its activity, creates receptor binding pockets and enzyme active centres.
Crystallography is one of the few methods that allows the structural determination of such macromolecules with atomic precision. By studying the interactions of crystallised molecules by means of high energy X-rays, it is possible to pinpoint the location of atoms and their bonds in a given molecule of interest.
During this course, we will set up a protein crystallisation experiment, learn the operation of high-precision pipetting robots, and cryogenically freeze protein crystals to prepare them for X-ray data collection at the synchrotron.
5. How Do Cells Know What to Do?
Nevenka Radic and Clara Borràs (Signalling and Cell Cycle Laboratory)


Cells are in permanent contact with their changing environment. This means that they have to react to these changes in order to regulate proliferation, survival and migration but also to prevent possible l damage. In this context, cells have developed several mechanisms that allow them to integrate and interpret external signals to produce appropriate responses. These mechanisms, called “signalling pathways”, consist of a series of chemical modifications that occur inside the cell when a certain stimulus is received. One of the crucial modifications in signalling pathways is phosphorylation, during which proteins are phosphorylated by other proteins—the so-called kinases. Each kinase selectively phosphorylates a specific group of proteins, and in this way confers specificity to the signalling pathway.
In our laboratory, we study a particular kinase called p38 MAPK. This kinase is activated under different cellular stress situations and it plays a critical role in inflammation, cell growth, proliferation, and differentiation, and cell death. Deregulation of this specific signalling pathway can lead to diseases such as cancer. Therefore, we study how p38 MAPK inhibition couldhelp to treat different aspects of tumour formation.
In this course, we will learn about the p38 MAPK signalling pathway and get to know the main laboratory techniques used to study it. We will also use cultured cancer cell lines to see how p38 MAPK influences diverse stages of tumour formation.
6. Towards “greener” synthesis of small drugs
Marina Bellido (Research Unit on Asymmetric Synthesis)

Currently one of the main concerns of pharmaceutical companies is the huge volume of by-products generated during the synthesis of drugs. As a result, companies are exploring ways towards more sustainable chemistry and waste reduction. Paul Anastas first defined the “Green Chemistry” field in the 1990s as "the design of chemical products and processes that reduce or eliminate the use and generation of hazardous compounds." The strategies in this field range from changing to greener solvents to using catalysts, for instance.
This course introduces the concept of sustainability in chemistry. In particular, we will focus on the first synthesis of Ibuprofen and how it has been improved over the years. As a practical course, we will synthesize this widely used analgesic and anti-inflammatory drug. Additionally, you will get to know standard laboratory techniques and instruments we use for the development of bioactive molecules.
Venue
Crazy About Biomedicine course will take place at the IRB Barcelona facilities.
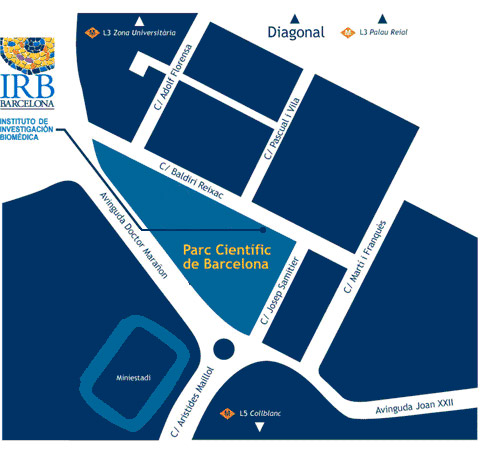
Institute for Research in Biomedicine (IRB Barcelona)
Parc Cientific de Barcelona
C/ Baldiri Reixac, 10
08028 Barcelona

