Organized by IRB Barcelona in collaboration with the Fundació Catalunya La Pedrera.
This year's tutors are Daniela Romão, Jery Joy, Miguel Rovira, Mate Maus, Jorge Garcia, Mireia Muriel, Laura Gonzalez, Nevenka Radic, Joan Antoni Matarín, Albert Cabré, Diego Gallego, Lara Barrio, Joel Paz, Marta Lovera, Elena Melendez, Raquel Bernad, Cristina Figueras, Maria Salvany, Marta Puerto.
Presentation
Objectives
Crazy About Biomedicine is a course directed at students in their first year of baccalaureate who wish to explore some of the exciting discoveries being made in the life sciences. Through this course, students will have a chance to deepen their knowledge of scientific theory and techniques in the field of biomedicine. They will work alongside our young researchers to get a taste for what doing science in a top international research institute is like, gain some hands-on experience in the latest cutting-edge methodologies, and position themselves for a potential career in the life sciences.
Course Description
A year-long workshop in the life sciences for high-school students organized by IRB Barcelona within the series "Crazy About Science" of the Fundació Catalunya La Pedrera.
This course includes a combination of theoretical lectures and practical hands-on experimental activities, which take place on 18 Saturdays throughout the year. Presented by IRB Barcelona PhD students and postdocs, the course will cover 12 hot scientific topics, ranging from cell and molecular biology to structural and computational biology, and chemistry. In the first ‘semester’ (January-April), the first three Saturdays will be devoted to these general lectures for all participants. During the following six sessions, small groups will enter the labs for hands-on practical experience. This schedule will then be repeated with six new research topics in the second semester (May-November). Participants must commit themselves to attending the whole course.

Course language
All lectures and practical sessions will be conducted in English.
Course dates and times
The course will run from January to November 2019, 10.00-14.00h.
SEMESTER I
- Fri. 11 January 2019: Inauguration at Mon Sant Benet- Sant Fruitós del Bages
- Sat. 12 January: Talk 1-2
- Sat. 19 January: Talk 3-4
- Sat. 2 February: Talk 5-6
- Sat. 16 February: Practical session 1
- Sat. 2 March: Practical session 2
- Sat. 16 March: Practical session 3
- Sat. 30 March: Practical session 4
- Sat. 6 April: Practical session 5
- Sat. 27 April: Practical session 6
SEMESTER II
- Sat. 18 May: Talk 1-2
- Sat. 25 May: Talk 3-4
- Sat. 8 June: Talk 5-6
- Sat. 14 September: Practical session 1
- Sat. 28 September: Practical session 2
- Sat. 19 October: Practical session 3
- Sat. 26 October: Practical session 4
- Sat. 9 November: Practical session 5
- Sat. 23 November: Practical session 6
Course fees
There is a fee of 150 euros to contribute to administrative and insurance costs for this programme, payable directly to the Fundació Catalunya La Pedrera.
Course location
Institute for Research in Biomedicine (IRB Barcelona)
C/ Baldiri Reixac, 10
08028 Barcelona
Who can apply
This course is directed toward students in the first year of their baccalaureate (only), who have a special interest and talent in the fields related to the life sciences (primarily biology and chemistry).
Students may apply to a maximum of 2 programmes within the "Crazy About Science" series, and can participate in only one.
How to apply
 Applications have to be submitted from here, starting on 17 September 2018.
Applications have to be submitted from here, starting on 17 September 2018.
Interested students must fill in the online application form and include a letter of motivation. A letter of recommendation will be requested directly from two of their teachers, who should know the applicant well. If the applicant has recently changed school then the letters of recommendation should be requested from his/her former teachers. The deadline for registration is 25 October 2018.
The course is open to a total of 24 students. Candidates will be selected on the basis of their academic record, teacher recommendations and motivation to participate. A short list of candidates will be invited for interviews with the scientific organizers in November after which the final selection will be made. Students will be informed of the outcome by the first week in December. The students selected to participate and their parents/guardians will be asked to sign a letter of commitment to attend all sessions.
Collaborators
Facebook: @LaPedrera.Fundacio
Twiter: @PedreraFundacio
Instagram: #bojosperlaciencia
Facebook: @LaPedrera.Ciencia
Twiter: @PedreraCiencia
Instagram: @lapedrera_ciencia

![]()
If you have any question, please contact us at: irb_outreach@irbbarcelona.org
Important dates
 25 October 2018: Application deadline
25 October 2018: Application deadline- 31 October - 22 November 2018: Short-listed candidates contacted
- 12-22 November 2018: Interviews
- 27 November - 2 December 2018: Selected students contacted
- Fri. 11 January 2019: Inauguration at Món Sant Benet –Sant Fruitós del Bages
- Sat. 12 January 2019: Course begins
- Closing Ceremony - To be determined
Programme
SEMESTER 1
1. Drosophila melanogaster: Getting to know yourself through the fly (Part I)
Daniela Romão and Jery Joy (Development and Growth Control Laboratory)


Drosophila melanogaster has been increasingly studied by the science community for over a century as a model organism for genetic investigations. It’s very commonly referred as a powerful genetic tool to unravel new discoveries about animal development and behavior, neurobiology, metabolism and common human diseases such as Parkinson or cancer.
Moreover, it’s more like us than you probably think. “Yeah…me and Drosophila? Lots of things in common” is probably not the most common thought on our heads when we hear out this model organism. Nonetheless, it is true. On a molecular level, the fruit fly has many similar features and conserved pathways when compared to humans, and 60% of a group of identified genes that are mutated, amplified or deleted in diverse human diseases are known to have a counterpart in Drosophila. Plus, they are easy to culture and maintain and they possess a short life cycle with lots of offspring that allows research to be conducted in a thoroughly and time saving manner. Furthermore it has a small genome size and a low number of chromosomes, which facilitates the study and manipulation of the genomic content.
In this course you will learn how to take your first steps in fly genetics. You will learn how to cross them, how to find and recognize genetic markers, balancers and other genetic tools that are used in a Drosophila lab every day. During the practical course you will also be introduced to the anatomy of the fly (in adult and larval stage) and have the opportunity to see different fluorescent makers under the microscope. A part from these tasks, you will also learn how to perform in vivo dissections and do their respective immunohistochemistry.
2. Strategies to understand ageing and senescence
Miguel Rovira and Mate Maus (Cellular Plasticity and Disease)

 Ageing is characterised by a gradual deterioration of physiological function, and it is considered the primary risk for human pathologies such as cancer or cardiovascular disorders. One of the main hallmarks of ageing is cellular senescence, the phenomenon by which normal cells cease to divide. Senescent cells accumulate in the organism over time, secreting pro-inflammatory molecules and contributing to ageing-related diseases.
Ageing is characterised by a gradual deterioration of physiological function, and it is considered the primary risk for human pathologies such as cancer or cardiovascular disorders. One of the main hallmarks of ageing is cellular senescence, the phenomenon by which normal cells cease to divide. Senescent cells accumulate in the organism over time, secreting pro-inflammatory molecules and contributing to ageing-related diseases.
There is an increasing interest in clinical medicine to better identify and target senescent cells, as their elimination delays and ameliorates some ageing-associated diseases, and can even extend longevity.
Students will acquire hands-on experience using different techniques to induce cellular senescence in normal cells. They will learn state of the art techniques to specifically target senescent cells, analysing in vitro and in vivo samples. Finally, they will also have the opportunity to perform classical protocols to detect senescent cells such as the SAβgal staining. These techniques will help us to understand, identify and target senescent cells, which is a promising therapeutic approach in clinical medicine.
3. Stepping into the world of proteins
Jorge Garcia and Mireia Muriel (Structural biology of protein & nucleic acid complexes and molecular machines)

 Proteins form the very basis of life. They regulate a variety of activities in all known organisms, from replication of the genetic code to oxygen transport, and are generally responsible for regulating cellular machinery and determining the phenotype of an organism. Proteins accomplish their tasks in the body by three-dimensional (3D) interactions between various substrates. The functional properties of proteins depend upon their 3D arrangement. The 3D structures arise because particular sequences of amino acids in a polypeptide chain fold to generate from linear chains to compact domains with specific organizations. The folded domains can serve as modules for larger assemblies or provide specific catalytic or binding sites.
Proteins form the very basis of life. They regulate a variety of activities in all known organisms, from replication of the genetic code to oxygen transport, and are generally responsible for regulating cellular machinery and determining the phenotype of an organism. Proteins accomplish their tasks in the body by three-dimensional (3D) interactions between various substrates. The functional properties of proteins depend upon their 3D arrangement. The 3D structures arise because particular sequences of amino acids in a polypeptide chain fold to generate from linear chains to compact domains with specific organizations. The folded domains can serve as modules for larger assemblies or provide specific catalytic or binding sites.
Various technologies, such as X-ray crystallography, are used to disclose protein structures. This particular technique calls for the use of crystals, a material whose constituents (in this case proteins) are arranged in an ordered pattern that extend in all three spatial dimensions. Crystals subjected to this technique, scatter the x-rays, and the information collected can help to infer the protein structure.
In this course, we will use the bacteria Escherichia coli to produce a protein of interest. We will use this same protein to make crystals and observe how a real protein crystal looks like!
4. Targeting cell signaling pathways to treat cancer
Laura Gonzalez and Nevenka Radic (Signalling and Cell Cycle Laboratory)

 Cells have to constantly deal with changes in their extracellular environment. It means that they have to respond efficiently to these changes in order to prevent damage but also they have to control proliferation, survival and migration. To do that, cells have developed several mechanisms that allow them to receive, integrate and interpret signals to produce the appropriate response. These mechanisms, called ‘signaling pathways’, are based in a series of chemical changes. One of the most important modifications is phosphorylation and proteins which are able to phosphorylate others are called kinases.
Cells have to constantly deal with changes in their extracellular environment. It means that they have to respond efficiently to these changes in order to prevent damage but also they have to control proliferation, survival and migration. To do that, cells have developed several mechanisms that allow them to receive, integrate and interpret signals to produce the appropriate response. These mechanisms, called ‘signaling pathways’, are based in a series of chemical changes. One of the most important modifications is phosphorylation and proteins which are able to phosphorylate others are called kinases.
Our laboratory is mainly focused in a particular protein kinase called p38 MAPK, which is activated under different stress situations and plays a critical role in inflammation, cell growth, proliferation, differentiation and cell death. However, when this pathway is dysregulated diseases such as cancer can arise. In that case, p38 MAPK inhibition could be beneficial to treat patients.
In this practical course we will provide an overview of the p38 MAPK signaling pathway and which are the main techniques we use in the lab to study it. We will use purified proteins and cultured cell lines to learn how p38 MAPK inhibitors work. Finally, we will explore which patients could benefit from them.
5. Chemical synthesis of bioactive small-molecules
Joan Matarín and Albert Cabré (Research unit on asymmetric synthesis)


Before a medicinal drug is considered suitable for patients, it has to go through several clinical phases just to ensure its activity and safety towards our body. Even before that, millions of compounds are chemically synthetized and tested just to select the compound with more potential activity. In the pharmaceutical industry, this entire process is known as the drug development process and in its early stage, organic chemistry assumes a crucial role. At the drug discovery phase, organic chemists modulate the chemical structure of a molecule that presents biological activity to fine-tune its further potency as well as its effects in our body. Therefore, organic chemistry is the key of success when new drug candidates are designed.
In this practical course we will synthesize benzocaine (Orajel®), which is a marketed drug used as a local anesthetic. While doing so we will learn how to design a synthetic route and the main techniques/instruments that organic chemists need to synthetize bioactive small-molecules.
6. Interacting is living, living is interacting
Diego Gallego (Molecular modelling and bioinformatics)

Have you ever wondered which are the fundamental principles that explain life? Our body, a fruit fly or a flower are all formed by cells. At the same time, cells (including bacteria and even archaebacteria) are formed by four big groups of molecules: lipids, carbohydrates, proteins and nucleic acids. However, if you mix these building blocks in vitro, you won't see a cell. Obvious, isn't it? A cell is much more than the sum of its parts. To understand the whole picture, you have to know how the parts (molecules) interact between each other. When you contemplate networks of interactions, you can understand very complex processes such as transcription, translation, mitosis, metabolism, signaling pathways, etc.
The correct interaction between any two molecules (e.g. a protein and DNA) depends heavily on their 3D structure. In turn, this structure is acquired through a process of folding, guided (again) by intra-molecular interactions. One of our main interests is understanding, modeling, and predicting the 3D structure, dynamics and interactions of nucleic acids.
In the practical course, students will see biomedicine from a computational perspective. They will be challenged with a real modeling problem that will lead them to cross the boundaries between structural bioinformatics, chemistry and physics. After this experience, the take-home message will be clear: interactions are the fundamental principle that allows us to understand each and every process of life.
SEMESTER 2
1. Drosophila melanogaster as a model for studying different types of growth: From development to tumorigenesis (Part II)
Daniela Romão and Lara Barrio (Development and Growth Control Laboratory)


Regulation of growth control is crucial for the normal development of any living organisms and for the maintenance of the homeostasis. Mechanisms such cell growth, differentiation, patterning, proliferation, programmed cell death and morphogenesis are tightly controlled during development in order to guarantee the correct formation of tissues, organs and the final anatomy and functionality of the adult animal. Drosophila is particularly well-suited for studying genetic regulation of growth control with a large variety of genetic tools available, and the ability to study tissue and cell specific defects in flies.
Besides its clear impact in development, growth regulation is also important in many genetic based diseases such as cancer. Cancer is a multi-hit process that involves mutations in oncogenes and tumor suppressor genes. This accumulation of mutations and epigenetic changes alter intrinsic cellular functions and cell-cell interactions in tissues. The conservation of signaling pathways and cellular regulatory systems between humans and flies makes the fruit fly the perfect model organism to study growth both in the context of development and of disease.
This course will be a second part of the “Drosophila melanogaster: Getting to know yourself through the fly” and because, by now, you will already be on your way to become a fly geneticist we will push you further and teach you how to mount dissected samples from both larvae and adult tissues. You will also learn how to proper image your samples using confocal microscope and process them using the correspondent statistics.
2. The role of microtubules in cell division and cancer
Joel Paz and Marta Lovera (Microtubule organization)

 Cellular cytoskeleton is perhaps one of the most important structures of the cell as it is involved in several functions as morphogenesis, cellular division or cell migration. It is composed by three members, actin filaments, intermediate filaments and microtubules. Microtubules are hollow cylindric polymers composed by tubulin dimers. Microtubules are very dynamic structures that constantly undergo episodes of polymerization and depolimerization which is known as dynamic instability. Microtubules are involved in key processes as intracellular transport of cargos (proteins, vesicles and even organelles) and in mitosis where they are in charge of the formation of the mitotic spindle and proper chromosome segregation. One of the hallmarks of cancer is uncontrolled cell division and one approach to treat cancer is to try to kill cancer cells. Compounds that target microtubules disrupting the normal cell division cycle and eventually lead to cell death have been proven to be one of the most effective cancer chemotherapeutic drugs available.
Cellular cytoskeleton is perhaps one of the most important structures of the cell as it is involved in several functions as morphogenesis, cellular division or cell migration. It is composed by three members, actin filaments, intermediate filaments and microtubules. Microtubules are hollow cylindric polymers composed by tubulin dimers. Microtubules are very dynamic structures that constantly undergo episodes of polymerization and depolimerization which is known as dynamic instability. Microtubules are involved in key processes as intracellular transport of cargos (proteins, vesicles and even organelles) and in mitosis where they are in charge of the formation of the mitotic spindle and proper chromosome segregation. One of the hallmarks of cancer is uncontrolled cell division and one approach to treat cancer is to try to kill cancer cells. Compounds that target microtubules disrupting the normal cell division cycle and eventually lead to cell death have been proven to be one of the most effective cancer chemotherapeutic drugs available.
In this practical course, we will learn how a mitosis occurs in vivo, what are the different phases of mitosis, what is the role of microtubules in this process and how can we treat cancer focusing on microtubules. For this purpose, we will use cultured cell lines treated with several chemotherapeutic drugs used in cancer therapy and see how they affect cell division with the help of fluorescence light microscopy techniques.
3. Manipulating cellular plasticity
Elena Melendez and Raquel Bernad (Cellular Plasticity and Disease)

 The concept of cellular plasticity has gained great relevance during the last years in the context of cancer and tissue repair. Cellular plasticity allows adult cells to regress to stem cell-like states through de-differentiation pathways.
The concept of cellular plasticity has gained great relevance during the last years in the context of cancer and tissue repair. Cellular plasticity allows adult cells to regress to stem cell-like states through de-differentiation pathways.
It is possible to convert differentiated cells into pluripotent stem cells (induced pluripotent stem cells or iPSCs) by the simple expression of four transcription factors. These iPSCs are functionally equivalent to embryonic stem cells (ESCs), which are derived from the developing blastocyst and can divide indefinitely while maintaining the capacity to differentiate into any cell type of the organism.
There is an increasing interest in better understanding how these transitions occur both in vitro and in vivo and how they can be manipulated. This knowledge will be directly applied in regenerative medicine to improve current medical treatments.
Students will learn the basic techniques to culture differentiated and pluripotent stem cells and they will be trained to induce the transition between cellular states. Finally, they will have the opportunity to perform in vitro assays that will help us to recognize the ultimate state of pluripotency.
4. In vivo experimental approach to study breast cancer bone metastasis
Cristina Figueras (Growth control and cancer metastasis)

In a primary tumor, many cells are constantly trying to enter into the circulation, travel through the bloodstream and arrive to a new organ to form a secondary tumor. This process is called metastasis and is very inefficient. However, tumor cells sometimes acquire properties that give them the ability to succeed and form a metastasis, which usually is fatal for cancer patients.
Metastasis is a very complex process, that is why we need different tools and techniques for its study. Eventhough in vitro experimentation gives us the possibility to study different aspects of tumor cells behavior inside the lab, in vivo experimentation allows a deeper understanding of how these tumor cells develop in their natural environment.
In our lab we combine all these techniques in order to study breast cancer metastasis. We use human cancer cell lines and animal models to unravel the role of specific genes in breast cancer bone metastasis.
In this course we will learn how cells are cultured and labeled in the lab, the techniques that we use to inject them into mice, how tumor growth can be monitored and the correct way to analyze the results obtained using in vivo models.
5. Targeting the Tumour Microenvironment in colorectal cancer metastasis
Maria Salvany (Colorectal cancer laboratory)

Colorectal Cancer (CRC) is the most common cancer and the second leading cause of cancer-related deaths in Spain, according to the AECC (Asociación Española Contra el Cáncer). CRC produces cancers in the large intestine through a multi-step process that, like many other types of cancer, starts with the acquisition of mutations in key genes. These mutations then go on to make cells proliferate without control. CRC can be easily treated if detected early. However, we still need to find effective treatments for the most advanced stages of cancer, particularly for metastasis—the final and deadliest step of cancer, which in the case of CRC occurs mainly in the liver.
The great potential of cancer cells resides not only in their capacity for mass proliferation but also in their ability to trick the healthy cells surrounding them. Fibroblasts, cells of the immune system, and blood vessels form what we call the tumour microenvironment (TME), which protects cancer cells from being removed and facilitates their invasion of other healthy organs. Our laboratory has recently shown that the TME is crucial for the aggressiveness of CRC and formation of metastasis in the liver. We are now channelling efforts into studying the potential of the TME as a way to block metastasis and into identifying novel treatments.
In this practical course we will provide an overview of several in vivo models and histological tools. Using these tools, we will discover and target those cells that form the TME of liver metastasis, with the aim to learn how to exploit them and stop the CRC metastasis.
6. The tridimensional genome
Marta Puerto and Paula Bujosa (Chromatin structure and function)


The molecule of DNA harbors the neccesary information to build a human being. Sourprisingly, each cell of our body has a total of 2 meters of DNA inside. Furthermore, the DNA is not naked, but is accompanied by a group of proteins forming the chromatin fiber. How is it possible to fit all this information in the tiny cell nucleus?
Researchers are trying to understand how the chromatin fiber is organized in the tridimensional space of the nucleus and how this organization affects the genome regulation. In terms of folding, the chromatin fiber can be found in two stages: euchromatin and heterochromatin. The euchromatin is a decondensed and open stage, with active genes expressing proteins that will perform specific cellular functions. The heterochromatin is a condensed stage, with silenced genes that will not be expressed into proteins. Therefore, the structural organization of the chromatin fiber affects which genes are switched on and wich genes are switched off.
In our laboratory, we are trying to understand the structure and function of the chromatin fiber, and for this pourpose we use Drosophila melanogaster as a model organism. In this course, we will use dissection techniques in flies to extract their polytene chromosomes and we will use stainning methods to label proteins that bind one of the two chromatin folding stages. Finally, we will observe preparations of euchromatin and heterochromatin under the microscope.
Venue
CRAZY ABOUT BIOMEDICINE will take place at the IRB Barcelona facilities.
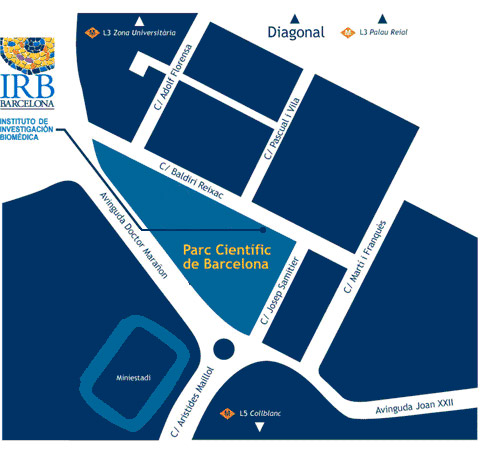
Institute for Research in Biomedicine (IRB Barcelona)
Parc Cientific de Barcelona
C/ Baldiri Reixac, 10
08028 Barcelona
(Campus de la Diagonal, Universitat de Barcelona)


