Research information
Background
Despite tremendous progress in therapeutic drug development, more than 80% of all human proteins remain beyond the reach of traditional drug discovery. Classical drug design mostly relies on synthetic compounds that block specific biochemical activities of proteins. These compounds are called “inhibitors” and are only suitable for proteins with accessible pockets, leaving some of the most relevant targets outside the reach of current drug development.
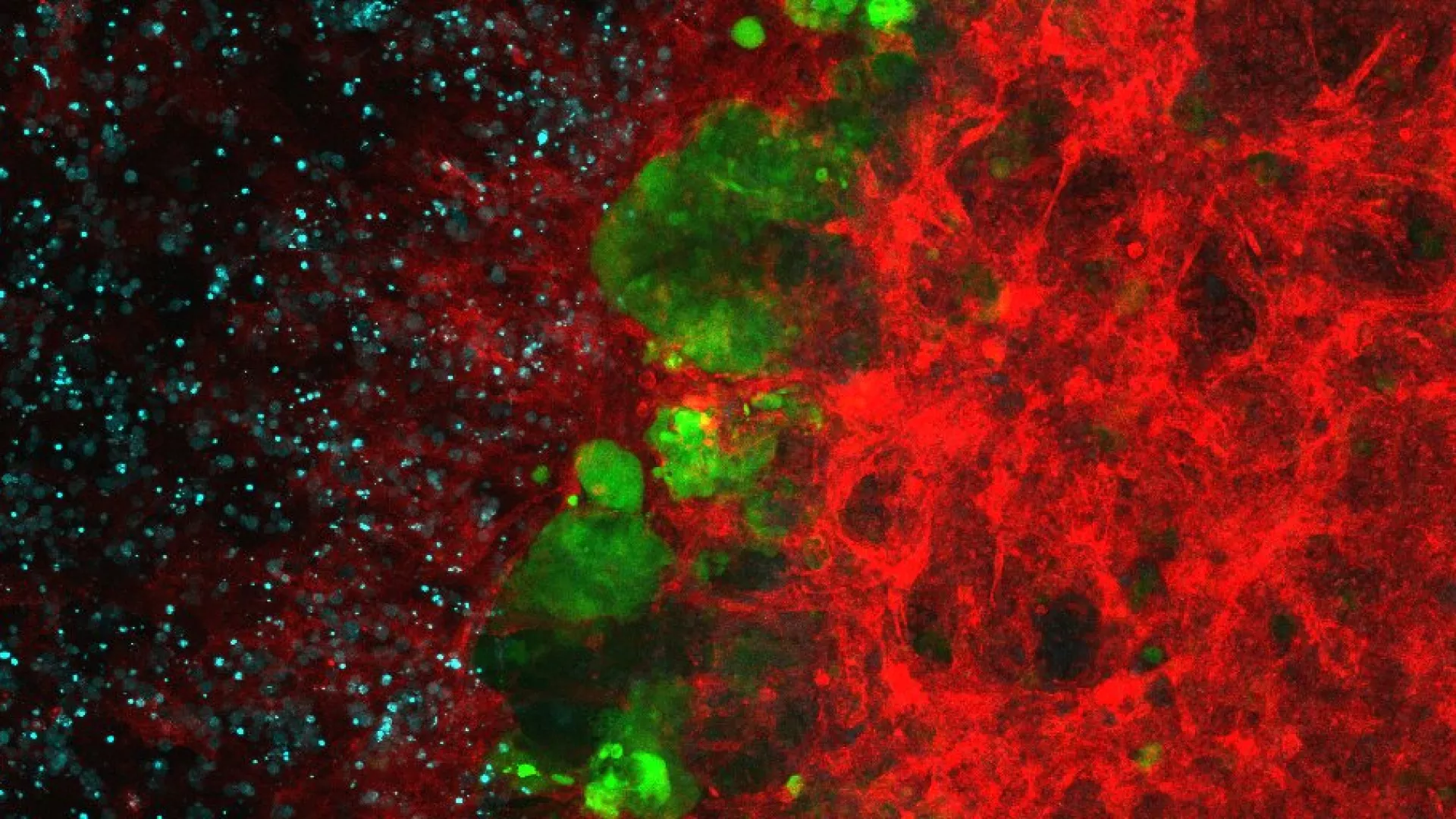
Targeted protein degradation (TPD) holds the promise to overcome these limitations. TPD is based on small-molecule drugs, generally called “degraders”, which induce proximity between a ubiquitin E3 ligase and a target protein of interest (neosubstrate). As a result, the target protein is ubiquitinated and then degraded by the proteasome. Modulation of protein abundance, selectivity and a catalytic mechanism of action are advantages of degraders over the classical inhibitors. Moreover, the inherent shift from “inhibition” to just “binding” has enabled the targeting of proteins so far considered undruggable.
The field of TPD has matured to a level where its generalizable nature is widely accepted, with dozens of targets shown to be amenable to targeted degradation. In the last years, TPD has stimulated intense academic research, but also, nearly all major pharmaceutical companies are pursuing in-house technology development and TPD drug discovery. Monovalent degraders are already a clinical reality, and the first clinical trials with modular degraders (PROTACs) started in 2019. Overall, the TPD technology represents a novel paradigm in drug discovery that holds the promise to eliminate otherwise undruggable proteins.
Research lines
Having TPD as a foundational basis, our research focuses on:
1. Drug discovery: we develop pioneering screening strategies to identify small molecules with therapeutic interest and we study the molecular mechanism of action. Conceptually, there are three types of degraders:
- Molecular glues (MGs): monovalent, non-chimeric molecules that induce highly cooperative de novo E3-neosubstrate interactions. They are not dependent on ligandable pockets but instead exploit protein-protein interfaces, inducing degradation of undruggable proteins such as transcription factors. This is exemplified by the clinically approved thalidomide and its analogs, collectively called IMiDs (immunomodulatory drugs).
- PROTACs (PROteolysis TArgeting Chimeras): heterobifunctional molecules which simultaneously bind target and E3 with dedicated warheads that are connected by a linker. The first wave of excitement in the field has been centered on PROTACs due to the relatively facile and scalable modular architecture. However, PROTAC design is intrinsically limited to targets ligandable via small molecules.
- Destabilizers: monovalent small molecules that induce indirect dimerizations between target and E3. They typically drive the target to a susceptible state (e.g. inducing biophysical changes that reveal a degron or preventing critical protective interactions), and then this state is recognized by an E3, prompting subsequent target degradation. One example is the clinically approved fulvestrant, which induces degradation of ER.
Lines of research:
- a. Expanding the toolbox for TPD.
- b. Beyond the current applications of inducing protein proximity to E3 ubiquitin ligases (novel PROTAC-like applications)
2. Molecular biology: we tackle exciting biological questions that, either benefit from the high kinetic resolution provided by TPD, or that involve E3 regulation dynamics.
Lines of research:
- a. Dysregulation of E3 ligase plasticity in disease (cancer and beyond)
- b. Oncoprotein degradation profiling
- c. TDP interplay with the immune system
Selected publications
Projects
Fundación Fero
Fundación Científica AECC. Referencia: IDEAS211100MAYO

Proyecto: PID2020-120110RA-I00, financiado por MCIN/ AEI/10.13039/501100011033

Fundación Científica AECC. Referencia: LABAE211700MAYO

European Research Council (ERC) mediante el Programa de Innovación e Investigación de la Unión Europea Horizonte Europa. Referencia: 101040046

Grup de Recerca consolidat (SGR-Cat 2021) del Departament de Recerca i Universitats. Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR). Referència: 2021 SGR 01279

The Mark Foundation for Cancer Research