Images
Participants


Contact

- An international study on the effect of circadian clocks in different tissues shows that there is a minimal network required for the control of glucose levels in the body. This finding has clear implications for diabetes and other age-related diseases.
Collaborative work by teams at the Department of Medicine and Life Sciences (MELIS) at Pompeu Fabra University (UPF), University of California, Irvine (UCI), and the Institute for Research in Biomedicine (IRB Barcelona) has shown that interplay between circadian clocks in liver and skeletal muscle controls glucose metabolism. The findings reveal that local clock function in each tissue is not enough for whole-body glucose metabolism as it also requires signals from feeding and fasting cycles to properly maintain glucose levels in the body. Understanding the components underlying glucose balance has clear implications for metabolic diseases such as diabetes or other age-related disorders I.
Circadian clocks are present in virtually every cell in the body. They align biological processes to a 24-hour cycle to synchronize physical, mental, and behavioural changes. This process is supported by the central clock in the brain that synchronizes clocks in peripheral tissues. “Maintenance of circadian rhythms is related to general health when robust, but to disease when disrupted. So circadian disturbances can affect carbohydrate metabolism and induce diabetes-like abnormalities,” explains Dr. Pura Muñoz-Cánoves, senior author of the study at MELIS-UPF.
The study published today in Cell Reports demonstrates, surprisingly, that clocks in liver and muscle can keep time on their own in the absence of the central clock in the brain, although the strength of their rhythms is reduced.
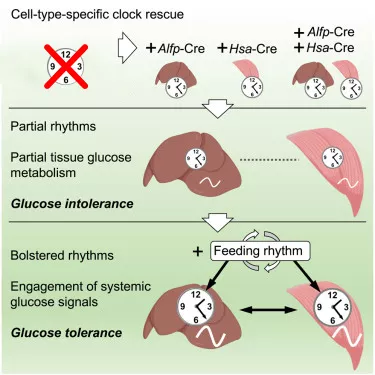
The study also found that in these conditions, glucose uptake and processing levels were altered. However, combining the clocks with feeding-fasting cycles improved the function of each of the clocks and restored glucose regulation in the combined system. This finding demonstrates that a daily feeding-fasting rhythm is key to the synergy of the liver and muscle clocks and to the restoration of glucose metabolic control.
Dr. Jacob Smith, a postdoctoral researcher at MELIS-UPF who co-led the study with Dr. Kevin Koronowski, commented: “Our study reveals that a minimal clock network is needed for glucose tolerance. The central clock, which controls daily feeding cycles, cooperates with local clocks in liver and muscle. The next step is now to identify the signalling factors involved in this interaction.”
“We believe this finding may hold promise for the treatment of human diseases such as diabetes, in which this liver-muscle network may be targeted for therapeutic gain, and for other age-related disorders,” adds Dr. Muñoz-Cánoves, who is now also a principal investigator at Altos Labs in San Diego.
The findings have been achieved using a 'clockless' mouse model, developed in Dr. Salvador Aznar Benitah ‘s lab at IRB Barcelona, in which they have restored clocks only in liver or skeletal muscle or combined clocks in both organs.
“This is a great example of how, by studying communication between peripheral tissues, one starts to understand the complex interplay of how systemic communication takes place. We are so thrilled to see how the daily coordination between the liver and the muscle was capable of sustaining systemic glucose tolerance, something that was completely unexpected by us,” explains Dr. Salvador Aznar Benitah, ICREA researcher and head of the Stem Cells and Cancer lab at IRB Barcelona.
This collaborative study was initiated in the laboratory of the late Dr. Paolo Sassone-Corsi at UCI and has been supported by the work of the laboratories headed by Dr. Selma Masri, Dr. Cholsoon Jang and Dr. Pierre Baldi at UCI.
As Dr. Aznar Benitah, commented, “this work is a testament to the collaborative and ground-breaking science that Paolo was known for.”
Reference article:
Liver and muscle circadian clocks cooperate to support glucose tolerance in mice
Jacob G. Smith*, Kevin B. Koronowski*, Thomas Mortimer, Tomoki Sato, Carolina M. Greco, Paul Petrus, Amandine Verlande, Siwei Chen, Muntaha Samad, Ekaterina Deyneka, Lavina Mathur, Ronnie Blazev, Jeffrey Molendijk, Arun Kumar, Oleg Deryagin, Mireia Vaca-Dempere, Valentina Sica, Peng Liu, Valerio Orlando, Benjamin L. Parker, Pierre Baldi, Patrick-Simon Welz, Cholsoon Jang, Selma Masri, Salvador Aznar Benitah*, Pura Muñooz-Cánoves*, and Paolo Sassone-Corsi*
Cell Reports (2023) DOI: 10.1016/j.celrep.2023.112588
About IRB Barcelona
The Institute for Research in Biomedicine (IRB Barcelona) pursues a society free of disease. To this end, it conducts multidisciplinary research of excellence to cure cancer and other diseases linked to ageing. It establishes technology transfer agreements with the pharmaceutical industry and major hospitals to bring research results closer to society, and organises a range of science outreach activities to engage the public in an open dialogue. IRB Barcelona is an international centre that hosts 400 researchers and more than 30 nationalities. Recognised as a Severo Ochoa Centre of Excellence since 2011, IRB Barcelona is a CERCA centre and member of the Barcelona Institute of Science and Technology (BIST).