Images
Participants





Contact

Researchers at IRB Barcelona reveal that the protein NEK7 is relevant for the correct formation of neurons in the hippocampus, a region of the brain associated with memory.
Animals without NEK7 may also have defects in other other brain regions.
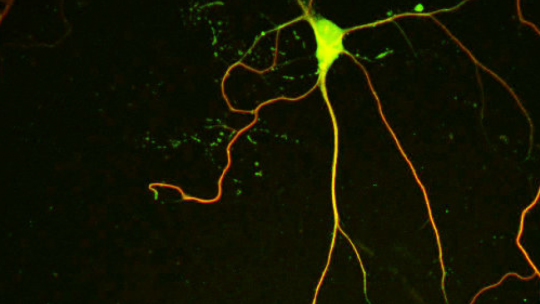
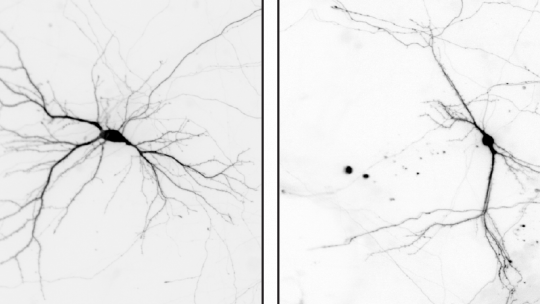
The protein NEK7 regulates neuron formation, as it is required for dendrite growth and branching, as well as the formation and shaping of dendritic spines. These are the main conclusions of a study published in Nature Communications and led by Jens Lüders at the Institute for Research in Biomedicine (IRB Barcelona), in collaboration with colleagues at the University of Barcelona and the Molecular Biology Institute of Barcelona (IBMB-CSIC).
It is firmly established that, during cell division, NEK7 regulates microtubules and centrosomes—structures that help separate chromosomes during mitosis. However, a function for this gene in neurons has never been reported. Microtubules are tiny filaments that shrink, lengthen, cluster and bend, depending on cell requirements. They are involved in cell mobility, cell division, and intracellular transport, among other functions.
The scientists have used in vitro and in vivo models to show that NEK7 is important for the correct formation of neurons in the hippocampus, a region in the brain involved mainly in the formation of memory. When researchers reduced the levels of NEK7, neurons did not form dendrites correctly. The dendrites became shorter and displayed fewer and improperly formed synaptic structures. The consequences that derive from the malformation of hippocampal neurons remain to be determined, but NEK7 deficiency results in a complex phenotype in mice, thereby suggesting that NEK7 has broader roles, potentially also in other brain regions.
Not just mitosis
Mitosis has been extensively studied, and most microtubule regulators have been identified. This knowledge led to the development of drugs that target microtubules and microtubule-regulating proteins to fight cancer. However, it is often overlooked that, microtubules also have other important functions in post-mitotic differentiated cells such as neurons.
Mitotic cells and neurons may indeed share more microtubule regulators than initially thought. By performing a genome-wide microarray analysis on differentiating neurons in culture, the researchers have identified so-called “mitotic microtubule regulators” that are strongly upregulated in neurons as they differentiate and thus presumably have key roles during this process. “It is of great importance to explore this premise, since not only will it lead to a better understanding of the function of the microtubule network in neurons but it might also help to predict cancer drug side effects,” says Francisco Freixo, former “la Caixa” PhD student at IRB Barcelona and first author of the study.
In neurons, for instance, microtubules help to define the shape of these cells and also serve as tracks along which many organelles and neurotransmitters are transported over long distances. “It is not surprising that some chemotherapeutic agents that affect microtubule functions in mitotic cells also have severe side effects in the nervous system,” explain Jens Lüders, head of the Microtubule Organization laboratory.
Microtubules determine neuron function and survival. However, to date, little is known about the regulators of the microtubule cytoskeleton in neurons. “In addition to understanding the potential side effects of the chemotherapeutic agents used to target mitotic cells, it is of paramount importance to have a better and more comprehensive knowledge of the identity of microtubule regulators in neurons, what they do, when and how they operate, and what happens when they are not present or when they are misregulated,” conclude the researchers.
Mutations in genes encoding microtubule cytoskeleton regulators are associated with neurodegenerative diseases and neurodevelopmental disorders. Examples include TAU, MAP1 and MAP2 associated with Alzheimer Disease, Doublecortin related with brain developmental diseases, and Spastin related to neuronal dysfunction. Future work is likely to reveal more so-called mitotic microtubule regulators that have important roles in the brain.
This study has been funded by grants from the Ministry of Economy and Competitiveness through ERDFs (MINECO/FEDER), the Government of Catalonia, and “la Caixa” Foundation.
See Jens Lüder's Meet Our Scientist VIDEO to get more information about the scientific research carried out in his laboratory.
Reference article:
Francisco Freixo, Paula Martinez Delgado, Yasmina Manso, Carlos Sánchez Huertas, Cristina Lacasa, Eduardo Soriano, Joan Roig & Jens Lüders
NEK7 regulates dendrite morphogenesis in neurons via Eg5-dependent microtubule stabilization
Nature Communications (2018) Volume 9, Article number: 2330
About IRB Barcelona
The Institute for Research in Biomedicine (IRB Barcelona) pursues a society free of disease. To this end, it conducts multidisciplinary research of excellence to cure cancer and other diseases linked to ageing. It establishes technology transfer agreements with the pharmaceutical industry and major hospitals to bring research results closer to society, and organises a range of science outreach activities to engage the public in an open dialogue. IRB Barcelona is an international centre that hosts 400 researchers and more than 30 nationalities. Recognised as a Severo Ochoa Centre of Excellence since 2011, IRB Barcelona is a CERCA centre and member of the Barcelona Institute of Science and Technology (BIST).